Origins of Innotox: Who Made It and Why
Key Points
First Liquid Botulinum Toxin – Innotox, created by Medytox in Korea, became the world’s first ready-to-use liquid botulinum toxin type A.
Efficiency & Consistency – Its liquid format eliminates reconstitution steps, reduces human error, and streamlines workflow in busy clinics.
Global Impact & Limits – While approved in Korea, Innotox is not FDA-approved in the USA, shaping debates on efficiency vs. flexibility in aesthetics.
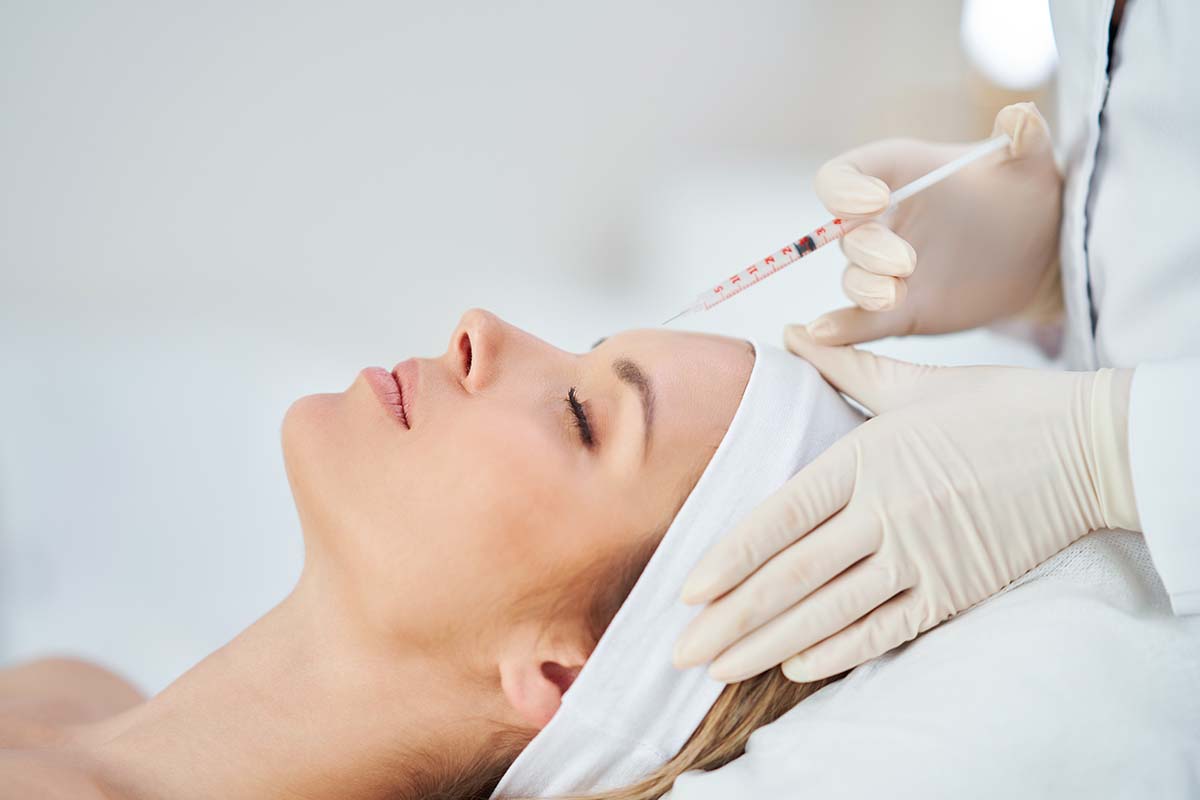
If you’ve ever worked with botulinum toxin products, the process feels familiar: a vial of powder, a syringe of saline, a careful mix, and only then the injection.
Innotox, however, took a different route.
Created by Medytox, a South Korean biopharmaceutical company, it was developed as a liquid solution that’s ready to use straight from the box.
Medytox is a key player in the Korea aesthetics market, known for products like Meditoxin and Neuronox, but Innotox became its standout innovation.
Approved in 2013 by the Korean MFDS, the product holds the distinction of being the first liquid botulinum toxin type A available anywhere.
That milestone marked not only technical progress but also Korea’s role as a driver of new anti-aging solutions.
Why make a liquid? Simple: every reconstitution step is a chance for inconsistency.
One staff member mixes differently from another; one clinic prefers 2.0 mL, another 2.5. By producing a factory-stabilized liquid, Medytox aimed to reduce human error and create a product that delivers the same concentration vial after vial.
The story doesn’t end in Korea.
Global eyes turned to this “liquid botox” when Allergan—maker of the iconic Botox—licensed Medytox’s liquid candidate (code-named MT10109L).
Allergan advanced it into late-stage clinical trials in the USA, raising expectations for a new alternative on the American market.
As of now, Innotox remains unapproved by the FDA, so clinics in the USA cannot legally order or buy online.
But its existence has changed how professionals think about workflow and formulation.
Liquid vs. Lyophilized: What’s the Difference?
To understand what sets Innotox apart, let’s compare the two main formats.
- Lyophilized toxins (freeze-dried powders): the global standard. They are highly stable, easy to ship long distances, and allow injectors to tailor dilution. But they always require careful reconstitution before use.
- Liquid toxins: already mixed and stabilized in solution at the factory. Instead of adding saline, you open the vial and draw directly into your syringe for injection.
Here’s an everyday analogy: lyophilized toxins are like powdered drink mixes—you add water and hope it tastes consistent every time. Liquid toxins are like bottled iced tea: ready to drink, same every time.
Each has pros and cons. Lyophilized gives flexibility and longer shelf stability.
Liquid eliminates preparation errors and saves time but fixes the concentration, limiting customization.
Both approaches can help smooth wrinkles and work for fine lines, but the format determines how injectors handle them.
Practices Choice Reason
So why do certain clinics favor Innotox? The short answer is efficiency.
High-volume practices can save time when each vial comes ready-made.
For those seeking reliable sources, platforms like https://tothebeauty.com/brand/innotox/ provide access to authentic Innotox products for professional use.
Staff don’t have to pause for mixing, so schedules run smoother. Because there’s no reconstitution, there’s less chance of uneven dilution or contamination.
That means results are more predictable across different staff members.
Some injectors also see Innotox as an alternative to standard lyophilized toxins, particularly when they want to simplify training for new staff.
With a liquid, the “did I add the right amount of saline?” step disappears, and focus can stay on placement technique.
Still, the liquid format comes with limits.
You can’t adjust the dilution to personal preference, and storage is stricter: refrigeration must be consistent.
And outside Korea, the ability to order legally is restricted by national approvals.
In the USA, for example, Innotox remains off-label and unavailable through authorized channels.
That hasn’t stopped people from trying to buy online, but regulators warn that gray-market toxins can be counterfeit or unsafe.
For patients, the end goal remains the same: smoother skin, fewer visible wrinkles, and an anti-aging solution that feels natural.
For clinics, the question is whether the workflow benefits of liquid outweigh the flexibility of powder.
The Bigger Picture: Toxins Are Not One-Size-Fits-All
The existence of Innotox doesn’t mean lyophilized toxins like Botox or Dysport are outdated.
Instead, it broadens the toolbox. Lyophilized products remain dominant globally because of their stability, long track record, and approval across dozens of markets.
Medytox’s liquid botox simply represents a different philosophy: improve clinical life by changing the solution format rather than the molecule itself.
Looking ahead, the debate isn’t about replacing one with the other but about understanding where each fits best.
For large, busy practices in Korea, a liquid may be ideal. For clinics that prize flexibility and global availability, lyophilized toxins remain the go-to.
In the bigger picture, Innotox shows how small changes—like shifting from powder to liquid—can ripple through practice management.
Whether or not regulators in the USA approve it in the future, this “alternative botox” has already influenced how professionals think about convenience, standardization, and innovation in anti-aging injections.
Conclusion
Innotox is a fascinating case study in formulation innovation.
Born in Korea, validated by global interest, and still awaiting wider approvals, it highlights the tension between workflow convenience and clinical flexibility.
For patients, the end results—smooth skin, softened wrinkles, and a fresher look for fine lines—remain the same regardless of format.
For clinicians, however, the choice between liquid and lyophilized is about efficiency, consistency, and regulatory access.
Just as cosmetics brands made in Seattle show, beauty solutions are not one-size-fits-all. They’re tools—and like any tools, the best one depends on the job, the setting, and the hands that use it.